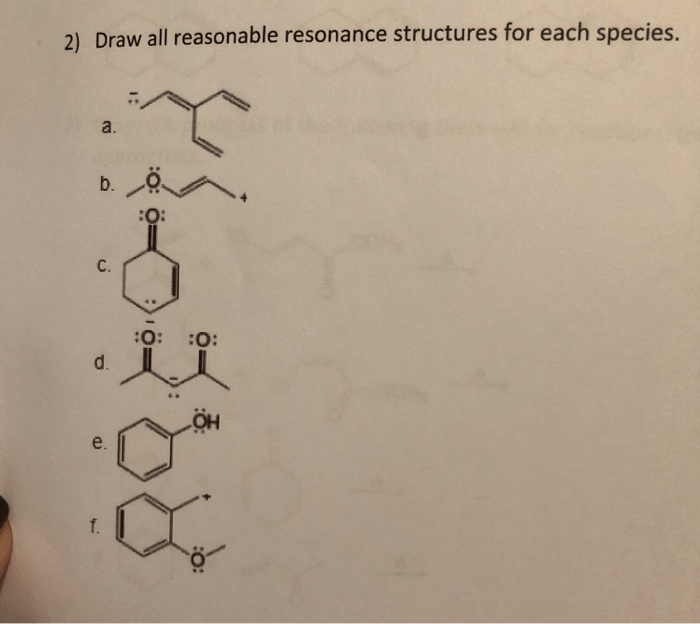
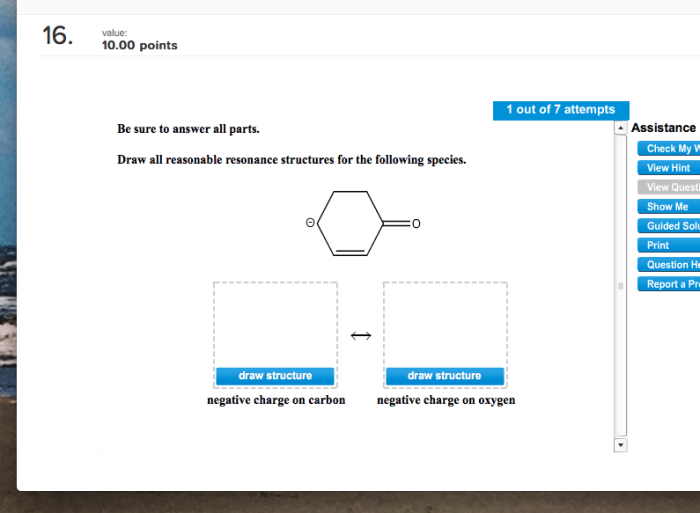
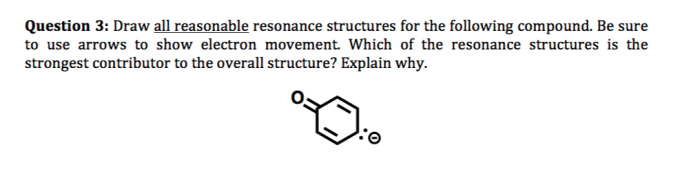
Draw all reasonable resonance structures for the following species introduces a topic of immense significance, delving into the intricacies of molecular structure and bonding. Resonance structures provide a powerful tool for understanding the behavior of molecules, enabling chemists to predict properties and reactivity with remarkable accuracy.
This guide will equip you with a comprehensive understanding of resonance structures, empowering you to navigate the complexities of molecular chemistry with confidence.
Resonance Structures
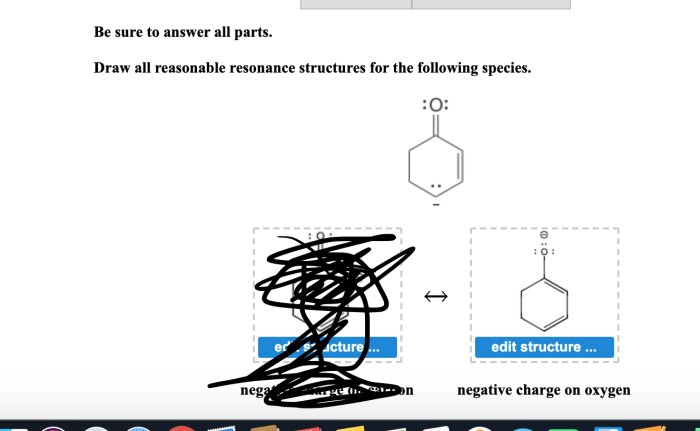
Resonance structures are alternative representations of the same molecule or ion that differ only in the placement of electrons. They are used to describe the delocalization of electrons within a molecule, which is a phenomenon that occurs when the electrons are not confined to a single atom or bond but are instead spread out over several atoms or bonds.
The concept of delocalization of electrons is important in understanding molecular structure and bonding. It can be used to explain the stability of certain molecules, the reactivity of certain functional groups, and the properties of materials.
Drawing Resonance Structures, Draw all reasonable resonance structures for the following species
To draw resonance structures, follow these steps:
- Identify the atoms or bonds that are involved in the resonance.
- Move electrons around to create different resonance structures.
- Use curved arrows to show the movement of electrons.
The resonance structures that are drawn should be equivalent in energy. This means that they should have the same number of unpaired electrons and the same overall charge.
Examples of Resonance Structures
Here are some examples of resonance structures for different molecules:
| Molecule | Resonance Structures |
|---|---|
| Benzene |  |
| Carbon dioxide |  |
| Ozone |  |
Applications of Resonance Structures
Resonance structures can be used to predict molecular properties, such as bond lengths and bond strengths. They can also be used to understand chemical reactivity. For example, resonance structures can be used to explain why certain molecules are more reactive than others.
Resonance structures are a powerful tool for understanding the structure and bonding of molecules. They are used in a variety of fields of chemistry, including organic chemistry, inorganic chemistry, and physical chemistry.
Question & Answer Hub: Draw All Reasonable Resonance Structures For The Following Species
What is the significance of resonance structures?
Resonance structures offer a deeper understanding of molecular structure and bonding, providing insights into electron delocalization and molecular stability. They are crucial for predicting molecular properties, reactivity, and behavior in various chemical processes.
How do I identify resonance contributors?
Resonance contributors are identified by their identical connectivity of atoms but differing arrangements of electrons. Look for structures that differ only in the placement of double bonds and lone pairs, while maintaining the overall atomic framework.
What is the role of curved arrows in drawing resonance structures?
Curved arrows are used to depict the movement of electrons as resonance structures interconvert. They indicate the shift of electrons from one atom or bond to another, providing a visual representation of the electron delocalization process.